Pharma Solutions

THE COMPLIANCE-SOLUTION FOR THE EU-FALSIFIED MEDICINES DIRECTIVE
In February 2019, new rules to counter the threat of falsified prescription medicines, enter into force with the EU Directive 2011/62. From that date, prescription medicines may no longer be provided to the European market if their packaging is not compliant, including a unique identifier.
INTEGRATION IN A SECOND STEP (OPTIONAL)
Start with BetterTrace Pharma to secure the continuity of your business and production. In a subsequent project BetterTrace Pharma can be fully integrated into your production line or be replaced by another solution.
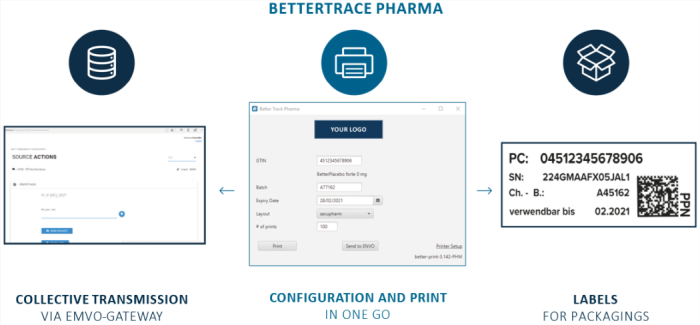
SIMPLE. FAST. COST EFFECTIVE.
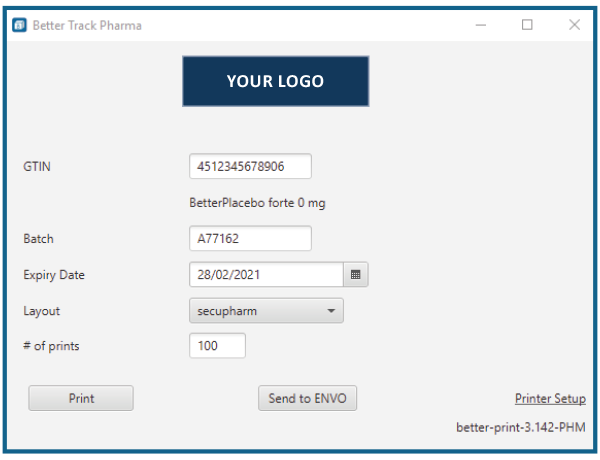
BetterTrace Pharma is an easy-to-use, fast-to-implement and cost-effective software solution that allows to print the information, which is required by the EU, on tamper-evident labels. At the same time, BetterTrace Pharma provides the required serial numbers in an export-file ready for transmission to the European Hub via the EMVO gateway.
PROVEN SOLUTION
BetterTrace Pharma is based on BetterTec Services GmbH‘s serialization solution BetterTrace, which has been proven in numerous projects in different industries and which now has been adapted to the specific requirements of pharmaceutical companies.
BUSINESS CONTINUITY FIRST!
BetterTrace Pharma is installed on an existing Windows PC or laptop – separated from your validated production line. Part of the delivery is a preconfigured label printer. The printing of labels takes place in a process which is independent from the production line. The fact that this process does not directly interfere with the validated production line might be an advantage. This makes BetterTrace pharma an ideal solution for all manufacturers who currently use labelling solutions already or who do not have the time to implement an integrated solution in time.
FAST IMPLEMENTATION AND EASE OF USE
Following the clarification of your needs, we develop the label templates for your company. Only after a test and your acceptance of the layouts the pre-configured system will be delivered to you. The solution is designed to be "plug & play" and can then be put into operation with little to none support. The simple and functional user interface allows intuitive operation after a short training session.
„We recommend planning for a six-month lead time! Please take into consideration that bottlenecks may arise both at EMVO and your on-boarding service providers.“
GET INFORMATION NOW!
If you are looking for a compliance solution to the EU Falsified Medicines Directive, please keep in mind that you must also pass the "Contractual Onboarding" and the "Technical Onboarding" for the European Medicines Verification Organization (EMVO) HUB. Both procedures may need some time and can only be performed stepwise!
The implementation of BetterTrace Pharma lasts about four weeks from the order placement and can be carried out in parallel to the Technical Onboarding to the EMVO HUB. Typically, the implementation time is as short as four to six weeks only. Contact us in case of any questions and we will support you to reach your compliance goal.
YOUR CONTACT PARTNER
Dr. Markus Gerigk
Address:
BetterTec Services GmbH
Pharma Solutions Unit
Heinrich-Rohlmann-Str. 8
50829 Köln (Cologne)
Germany
Phone:
+49 221 78306747
E-Mail:
mg@bettertec-services.com

ABOUT US
BetterTrace Pharma is a solution of BetterTec Services GmbH. Headquartered in Cologne, the company develops solutions for the serialization and personalization of products and documents. BetterTec Services GmbH serves its customers in the fields of distribution control, commissioning (pick, pack & ship), customer loyalty (CRM) as well as in the management of product recalls and warranty claims.
BETTERTEC Services maintains close, strategic partnerships with specialists for smart packaging solutions with integrated product security, as well as solution providers for the monitoring and enforcement of intellectual property rights.
